A major hurdle in the race toward cleaner, more affordable batteries has been the reliance...

A major hurdle in the race toward cleaner, more affordable batteries has been the reliance on expensive, hard-to-source metals like cobalt and nickel.
These elements power the cathodes in most lithium-ion batteries but carry environmental, ethical, and financial costs.
Now, researchers at McGill University, working with scientists from the U.S. and South Korea, have developed a new method to manufacture high-performance cathode materials that could remove the need for cobalt and nickel altogether.
Their breakthrough offers a scalable, energy-efficient way to produce ‘disordered rock-salt’ (DRX) cathodes, an alternative that has long shown promise but remained tricky to industrialize.
Molten salt process unlocks precision
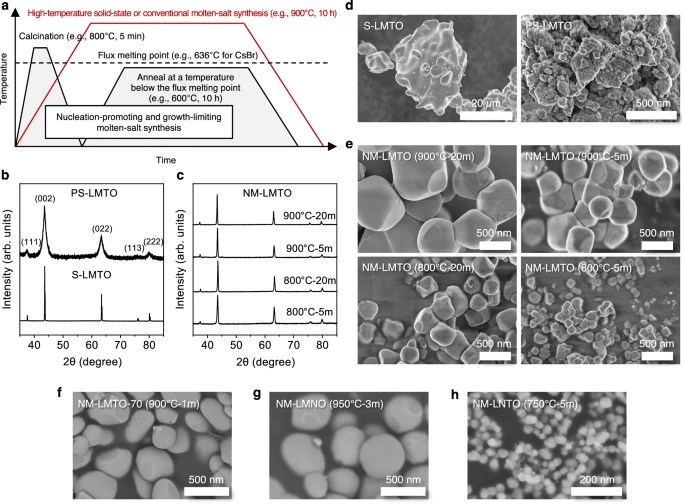
A two-step molten salt method lies at the heart of the team’s success.
By controlling the environment in which DRX particles form, researchers achieved fine-tuned nucleation followed by restricted growth, producing uniform, highly crystalline particles under 200 nanometers in size.
“Our method enables mass production of DRX cathodes with consistent quality, which is essential for their adoption in electric vehicles and renewable energy storage,” said Jinhyuk Lee, corresponding author of the study and Assistant Professor in McGill’s Department of Mining and Materials Engineering.
The approach eliminates the need for grinding or post-processing steps that previously made DRX manufacturing inefficient and inconsistent.
“We developed the first method to directly synthesize highly crystalline, uniformly dispersed DRX single particles without the need for post-synthesis grinding,” Lee added.
“This morphological control enhances both battery performance and the consistency of large-scale DRX cathode production.”
In testing, batteries built using the new DRX materials retained 85 percent of their capacity after 100 charge-discharge cycles, more than twice the durability of previous DRX particles made with conventional methods.
The particle uniformity and crystalline structure appear to be the key performance enhancers.
Crucially, DRX cathodes eliminate the need for nickel and cobalt, making them more environmentally and ethically sustainable.
Both metals are associated with controversial mining practices and volatile global supply chains. The McGill-led innovation could play a central role in reducing the industry’s dependence on them.
From lab to market
The project was carried out in collaboration with Stanford University’s SLAC National Accelerator Laboratory and the Korea Advanced Institute of Science and Technology (KAIST).
It also received backing from Wildcat Discovery Technologies, a U.S.-based battery company that’s now looking into scaling the new DRX production method.
“Acceptance of our work highlights both the fundamental insight and industrial potential of the method,” said Hoda Ahmed, the paper’s lead author and a Ph.D. student in McGill’s Department of Materials Engineering. “It shifts the field toward scalable manufacturing.”
With this new technique, the path toward next-generation lithium-ion batteries, cheaper, greener, and easier to mass-produce, has become far more realistic.
The study is published in the journal Nature Communications.